Nordimet®: simply designed with patients in mind

No button to press. Beneficial for patients with impaired manual dexterity1
Compact design to help minimise handling problems
Innovative click and gentle vibration confirming start and end of injection process
Viewing window indicates injection progress
Needle hidden from start to finish may aid with needle phobia
Ultra thin 29G, thin-walled, 5-bevel needle to minimise injection site discomfort
Nordimet® is activated in 2 simple steps:
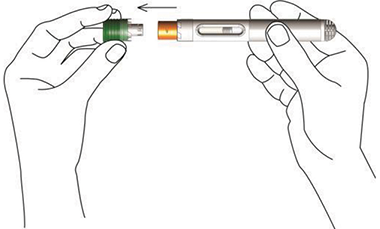
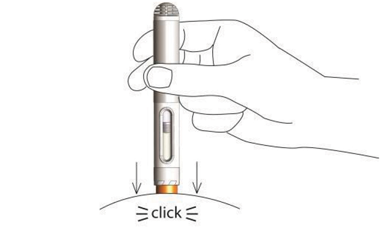
Press the needle shield against the skin. First click confirms start of injection. Second click confirms end of injection.
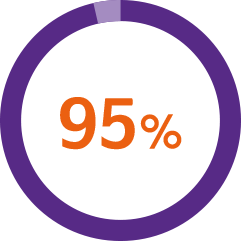
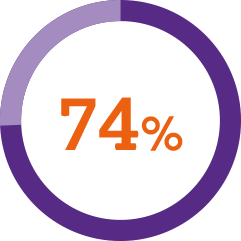
A usability study* demonstrated1


of patients successfully injected Nordimet® in a ‘worst-case’ environment**
of patients described the injection process as simple
Intuitive for patients new to self-injection1*
Simple for existing subcutaneous methotrexate users2**
Nordimet® is indicated for the treatment of:3
- - active rheumatoid arthritis in adult patients,
- - polyarthritic forms of severe, active juvenile idiopathic arthritis (JIA), when the response to nonsteroidal anti-inflammatory drugs (NSAIDs) has been inadequate,
- - severe recalcitrant disabling psoriasis, which is not adequately responsive to other forms of therapy such as phototherapy, psoralens and ultraviolet A (PUVA), and retinoids, and severe psoriatic arthritis in adult patients,
- - induction of remission in moderate steroid-dependent Crohn's disease in adult patients, in combination with corticosteroids and for maintenance of remission, as monotherapy, in patients who have responded to methotrexate.
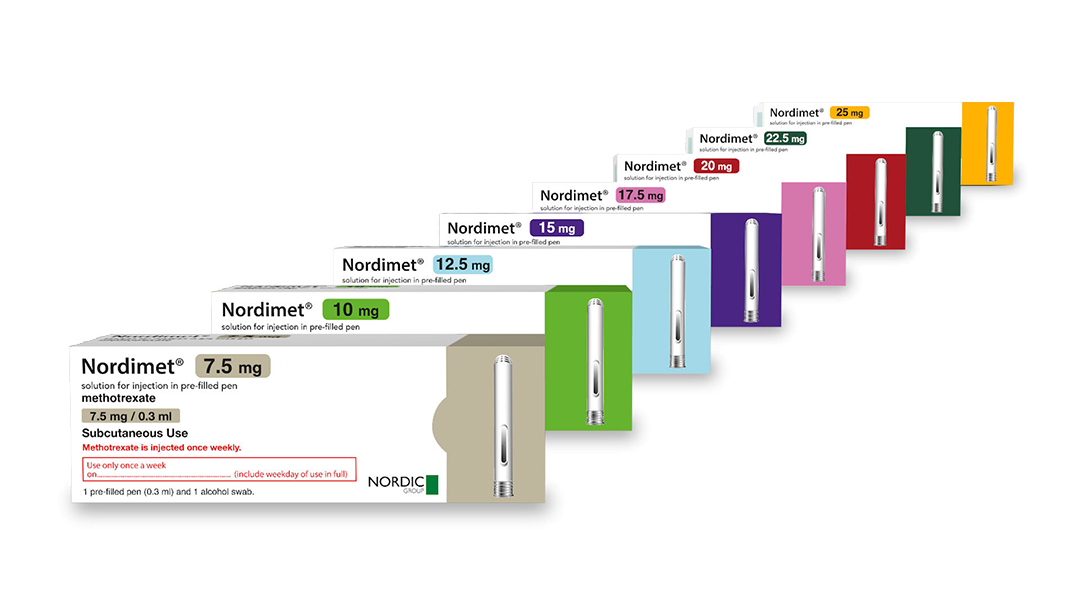
Nordimet®: a range of indications and presentations
8 presentations, in 2.5mg increments from 7.5mg to 25mg
-
The dose should be reduced to the lowest possible
effective maintenance dose, once the desired therapeutic
result is achieved -
The recommended initial dose is 7.5mg
once weekly, administered subcutaneously
- Hudry C. et al. Rheumatol Ther 2017;4(1):183-194.
- Homer D. Musculoskeletal Care 2019; 17:274-279
- Nordimet® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/2473/smpc#gref.