The importance of shared decision making
When considering new treatments, patient choice may lead to improved compliance.1
This is why patients and carers should be involved in making informed, shared decisions about their methotrexate therapy.2
In a patient preference study, 83% of patients chose Nordimet® versus a button-activated device.3
Patient preferences:


of patients with high hand disability successfully injected using the button-free Nordimet® device.4**
of participants were satisfied with the Nordimet® device and injection process.4**
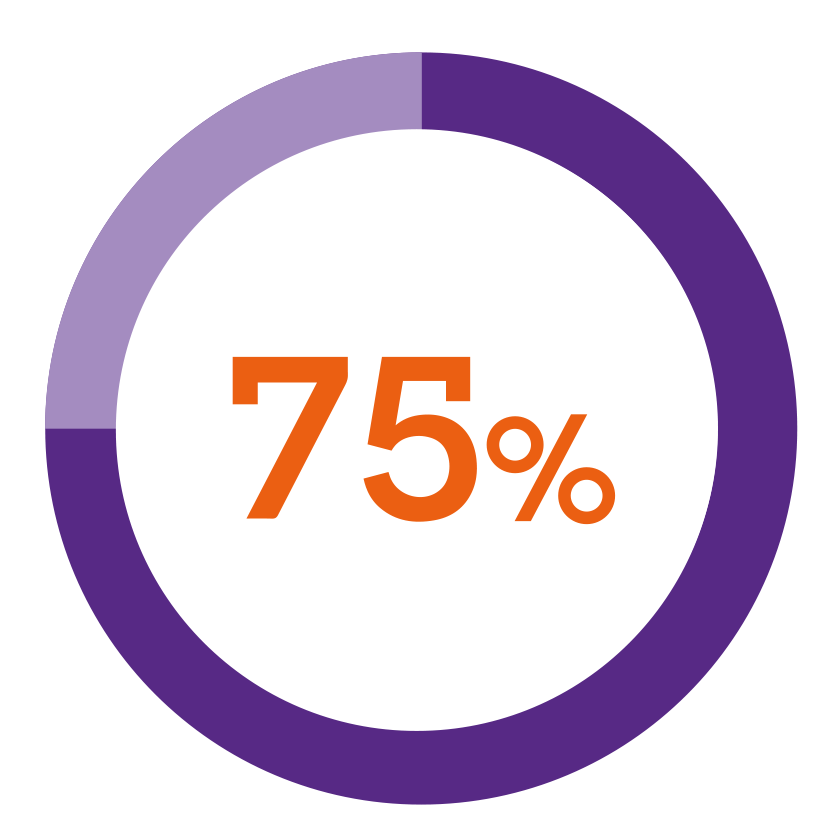
In a patient preference study5***
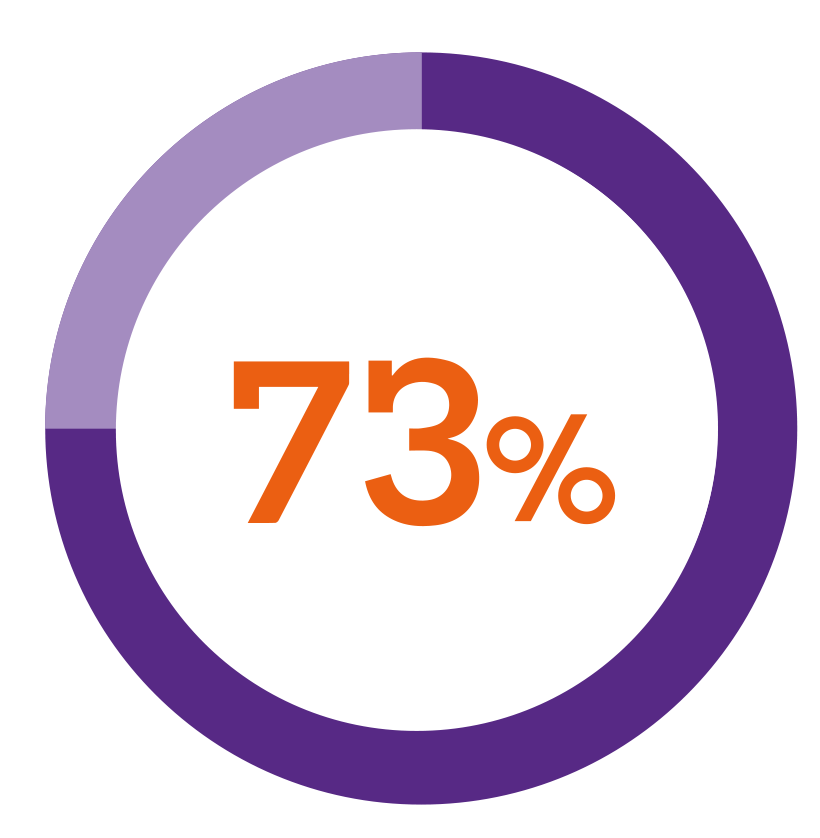
of patients using a button-activated device were interested in switching to Nordimet®
of patients using a button-activated device found Nordimet® easier to use
** Usability study of 42 rheumatoid arthritis patients and nurses/carers. High hand disability was defined as a score of ≥20 points, and low hand disability < 20 points.
*** Survey conducted by IPSOS Healthcare in France, Ireland, Spain and UK, involving face-to-face interviews with 100 patients with moderate to severe RA, using either Nordimet® (n=35) or a button-activated device (n-65) >1 month. The first phase included patient evaluation and rating of satisfaction with their current device. This was followed by a crossover phase, where they evaluated the other, unfamiliar device after receiving information, watching a demonstration and performing simulated injections.
References:
References:
- NHS England: Shared decision making (2022). www.england.nhs.uk/shared-decision-making/about/
- Administering subcutaneous methotrexate for inflammatory arthritis (4th ed.) (2022 ); Royal College of Nursing
- Larty J. (2022); Poster presented at BSR congress
- Hudry C. et al. Rheumatol Ther 2017;4(1):183-194.
- Zeitoun J-D., Morvan Y. Patient preference and adherence (2020); 14: 2177–2185.